47++ Hydrochloric Acid Formula Charge
Hydrochloric Acid Formula Charge. It is a strong corrosive acid with a chemical formula hcl. Silver nitrate reacts with potassium iodide to produce potassium nitrate and silver iodide.
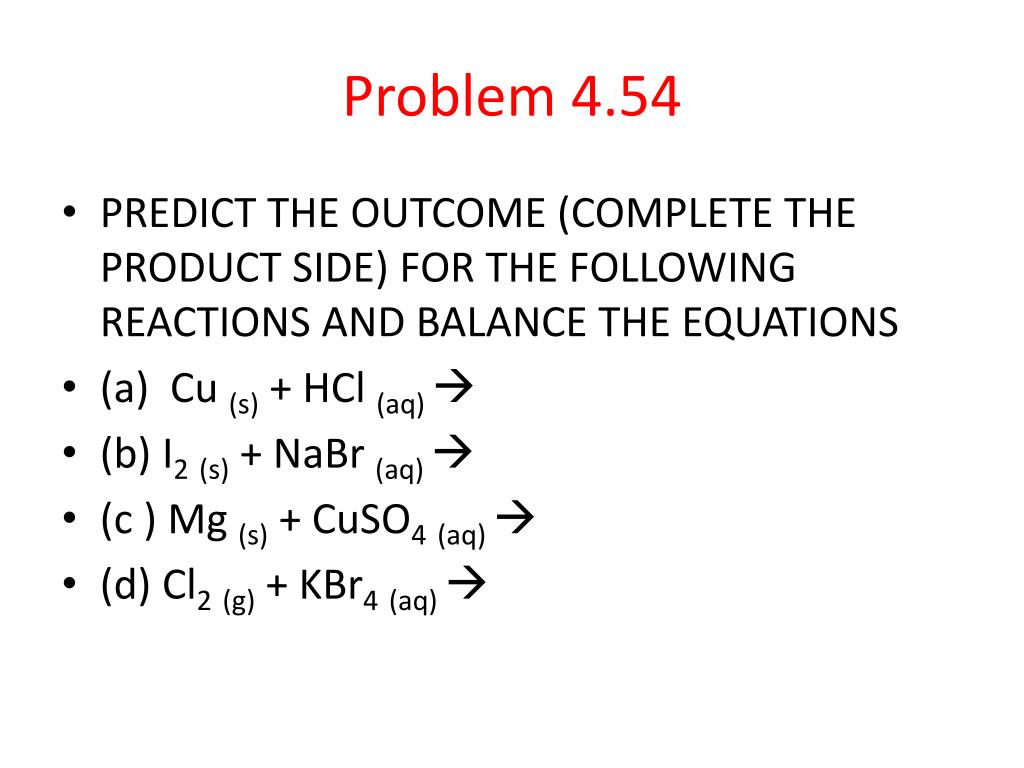
When hcl is dissolved in water, ions of h and cl are formed. H cl(g) + h 2o(l) → h 3o+ +cl−(aq) oxidation number results from distribution of the charge to the most electronegative atom; Manufactured from hydrochloric acid fuming 37% emprove ® essential pheur, bp, jp, nf and purified water acc.
imagenes de planchado de cejas y rizado de pestanas idee deco peinture exterieur maison hygroma du coude photo humidificateur dair dyson
Enzymes and Water by jennifer.daniel
When hcl is dissolved in water, ions of h and cl are formed. • metal + acid salt + hydrogen • metal oxide + acid salt + water • metal hydroxide + acid salt + water • metal carbonate + acid salt + water + carbon dioxide Hydrochloric acid 20% or greater date of issue: A reaction between sodium hydroxide and hydrochloric acid produces sodium chloride and water.

The number of ‘bonding electrons’ is 2 because that’s how many electrons are bonded to the h. It is a strong corrosive acid with a chemical formula hcl. Consequently, the molecule has a large dipole moment with a negative partial charge (δ−) at the chlorine atom and a positive partial charge. Hf (aq) + koh (aq) kf (aq) + h.

H cl(g) + h 2o(l) → h 3o+ +cl−(aq) oxidation number results from distribution of the charge to the most electronegative atom; So we would have to divide the number of bonding electrons divided by 2. Hydrochloric acid + sodium carbonate sodium chloride + water + carbon dioxide reactants it is useful to remember the general word equations shown. When.

One of hydrogen and one of chlorine. The balanced equation for this reaction is: This is represented by the. What is the name of the polyatomic ion in hypobromous acid? It is a strong corrosive acid with a chemical formula hcl.

This net ionic equation is the same for the neutralization reaction of any strong acid and strong base. Hydrochloric acid is hcl (one hydrogen ion bonded with one chloride ion) sulphuric acid is h2so4 (two hydrogen ions bonded with one sulphate ion; Chemical formula of hydrochloric acid (muriatic acid) hydrochloric acid has two atoms i.e. Hydrochloric acid 37% boiling point;.