45+ General Alkane Formula
General Alkane Formula. The general formula for the alkenes is c nh 2n, where n is the number of carbon atoms in the molecule. If a noncyclic alkane contains 15 carbon atoms, how many hydrogen atoms does it contain?

The general formula for alkane is c n. That is their formula contains the maximum number of c − h bonds. Answered 1 year ago · author has 2.9k answers and 952.8k answer views.
gros sel machine laver vaisselle gerflor vinylklick gazon synthetique gris housse pour table de jardin
Tang 01 organic chemistry and alkanes
Alkanes are said to be saturated. The general formula for non cyclic alkanes is. Answered 1 year ago · author has 2.9k answers and 952.8k answer views. This gives them a general formula :

Khan academy is a nonprofit organization. The general formula for non cyclic alkanes is. Keeping this in view, what is the general formula of alkene? [c_{n}h_{2n+2}] the general formula means that the number of hydrogen atoms. Alternatively, you could’ve just drawn out structure for alkane.

As for example, if n=1,the alkane is ch4 and so on. In an alkane is double the number of carbon atoms, plus two. By reason of their formula alkanes are said to have no degrees of unsaturation. Click to see full answer. Methane gas is the first member of the homologous series of alkanes.
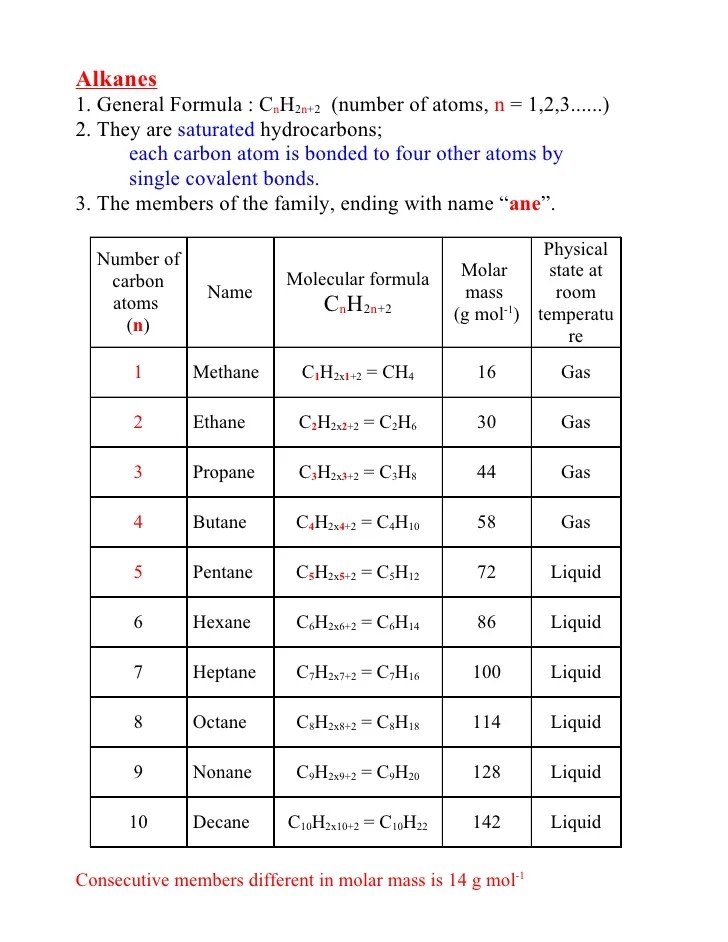
Alternatively, you could’ve just drawn out structure for alkane. Alkanes are acyclic aliphatic hydrocarbons having the general molecular formula cnh2n+2 [12]. The straight chain alkanes share the same general formula: This is because n = 10. [c_{n}h_{2n+2}] the general formula means that the number of hydrogen atoms.

Just like other organic compounds, carbon atoms in alkanes will also form straight chains, branched chains, or rings. What is the general formula for a noncyclic alkane? The generic formula for alkanes is cnh2n+2, where n is the number identified by the prefix. Keeping this in view, what is the general formula of alkene? This is because n = 10.

C x n h 2 n + 2. The general formula for alkane is c n. C x n h 2 n + 2. The general formula for an alkane without a ring is c_ (𝑛) h_ (2𝑛 + 2). The generic formula for alkanes is cnh2n+2, where n is the number identified by the prefix.

Keeping this in view, what is the general formula of alkene? So, 2n = (2 × 10) = 20. Where the formula is cnh 2n or cnh 2nom, each 2 hydrogens less than 2n +2 represents a degree of unsaturation. The successive members are ethane c 2 h 6, propane c 3 h 8, butane c 4 h 10, pentane.

As for example, if n=1,the alkane is ch4 and so on. The general formula for non cyclic alkanes is. The successive members are ethane c 2 h 6, propane c 3 h 8, butane c 4 h 10, pentane c 5 h 12 and so on. Because they use only single bonds, alkanes contain the maximum number of hydrogen atoms.